Improving student learning via active practice-oriented performance expectations in General Chemistry
Chemistry is an experimental science; consequently, extensive involvement with laboratory work provides an opportunity for learners to develop deeper connections between theoretical ideas and practical skills. This project aims to improve student learning of the core ideas and scientific skills needed to understand chemical phenomena. The project proposes a thoughtful design of novel in-class activities and assessments for the General Chemistry courses to meet its goal. The project will create a suite of active-learning in-class simulations and virtual laboratory experiences to promote data analysis and interpretation using available resources (i.e., virtual labs and simulations). All activities will have an assessment component to determine the impact of student learning activities and the potential benefit to students’ overall performance in the course. In 2022, the PI, Adrian Villalta-Cerdas, co-authored a complete set of novel learning outcomes and performance expectations for the General Chemistry sequence, thus facilitating the selection of the target learning goals for the assessments and activities to be produced in this project.
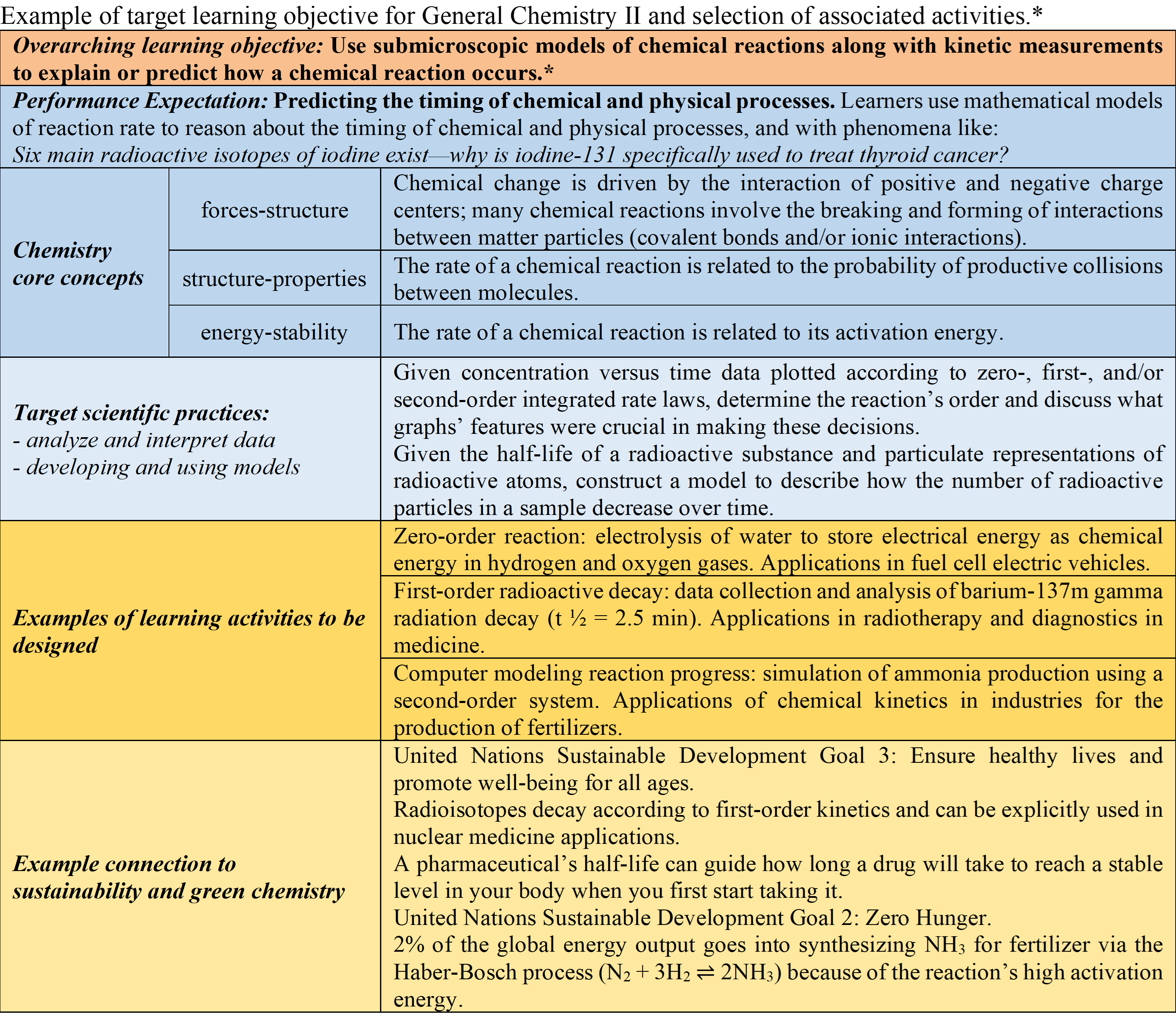
* Information based on Pazicni, S.; Morgan Theall, R. A.; Richter-Egger, D.; Villalta-Cerdas, A.; Walker, D. R. (2022). General Chemistry Learning Outcomes. The Center for Curriculum Redesign. Project funded by the Bill and Melinda Gates Foundation. Weblink.
Fostering STEM Success: Mapping, Analyzing, and Modeling for Success at SHSU
Science, technology, engineering, and mathematics (STEM) education is paramount in today's rapidly evolving global landscape. A strong foundation in STEM disciplines prepares students for rewarding careers and drives innovation in various industries. STEM skills are crucial to economic growth, technological advancements, and competitiveness in the global market. However, challenges in STEM education, particularly in the form of "choke" points or difficult points in the curriculum progression, may hinder student success and contribute to the attrition of STEM majors. High failure rates, difficulty in mastering complex concepts, and lack of effective instructional strategies are some factors that can lead to these choke points. Addressing these challenges is critical for retaining and graduating a skilled STEM workforce.
The research project herein aims to investigate the curriculum progression of STEM majors over ten years and identify the choke points to optimize educational outcomes for students who graduate from SHSU. By understanding the specific challenges that students face in STEM education, this research will improve retention rates and ensure a qualified workforce in these critical disciplines. By addressing the challenges in STEM education and identifying the "choke" points in the curriculum progression, we will help to optimize educational outcomes for students who graduate from SHSU. The products of this research will not only improve retention rates but also ensure a skilled and diverse workforce in the critical STEM disciplines, ultimately fostering innovation and economic growth.
Transition pathways between majors as students moved through the course sequence.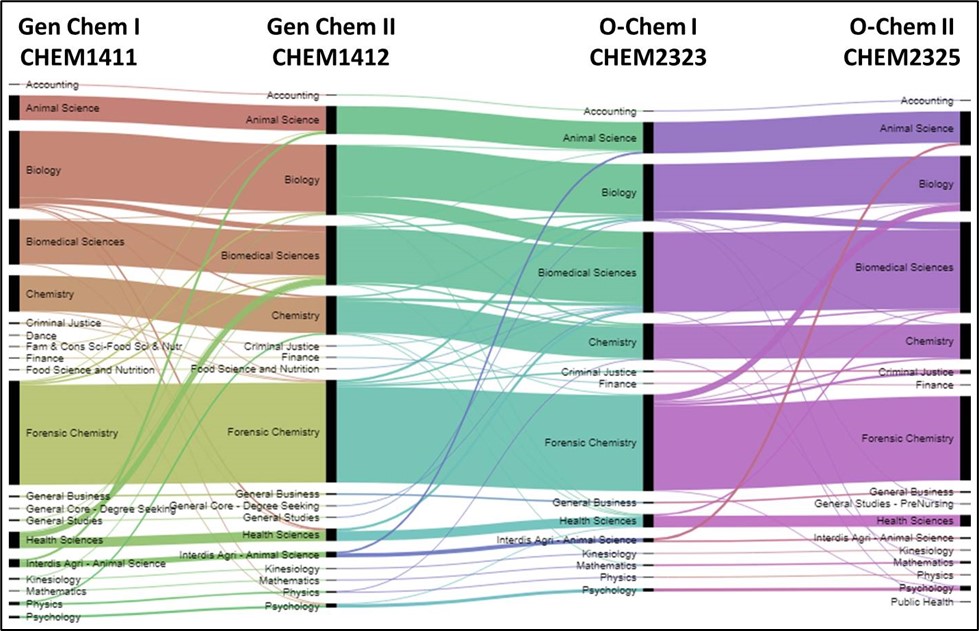
Grade transition pathways as students moved through the course sequence.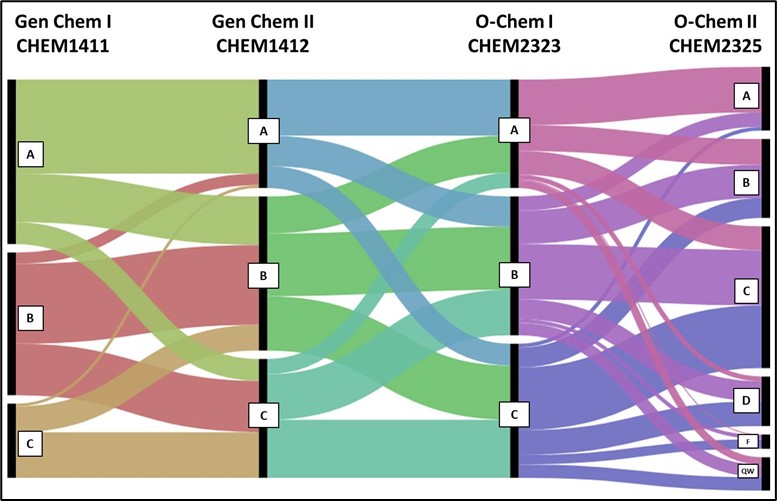
A Comprehensive Model for Improving the Success of STEM Majors through the STEM Center
The STEM Center at SHSU seeks to increase the number and quality of STEM graduates by establishing a strong foundation for learning using innovative teaching practices and supporting students in finding research and internship opportunities and building lifelong skills needed for advancement and leadership in STEM careers. The center is funded by the NFS award #1725674.
